* indicates required field
The Virtual Pooled Registry Cancer Linkage System (VPR-CLS) is a secure online service designed to:
- efficiently connect researchers performing minimal risk linkage studies with multiple U.S. population-based cancer registries;
- perform linkages utilizing a single cohort file, standard linkage software, and consistent matching algorithms;
- provide initial aggregate match count results to researchers; and
- streamline the process of applying for and tracking release of individual-level data on matched cases.
For a quick overview of the process, please see the VPR-CLS explainer video: here.
Coordinated by the North American Association of Cancer Registries (NAACCR) with funding from the National Cancer Institute, the VPR-CLS provides a single location to facilitate timely access to and use of high quality cancer surveillance data for minimal risk linkage studies. Use of an automated, standard linkage methodology and a streamlined application process significantly reduces the level of effort researchers and registries must dedicate to the linkage and approval process. The technology for the VPR-CLS has been developed by Information Management Services, Inc. (IMS), which also serves as the system's third party honest broker.
Requirements for Using the VPR-CLS
The VPR-CLS is designed to facilitate minimal risk linkage studies with multiple cancer registries. In order to utilize the VPR-CLS, the following requirements must be met:
- Study has (or will have) an existing cohort file that meets the following criteria:
- At least 75% of records contain 9-digit SSN, DOB and either First Name OR Last Name; OR
- At least 75% of records contain First Name, Last Name, DOB, Gender, and at least one of the following: 4-digit SSN, Phone #, OR Full Address (excluding P.O. Box)
- Study has a current IRB-approval, IRB exempt determination, or documentation of Not Human Subjects Research
- The study protocol includes linkage with cancer registries
- Study consent form includes linkage with cancer registries OR study has a specific waiver of informed consent to link with registries
Description of Phase I and Phase II Approach
The VPR-CLS consists of, and has been developed in, two distinct phases:
- Phase I I supports a standardized linkage with participating registries and release of provision of aggregate match counts (by registry and diagnosis year), allowing the researcher to prioritize registries from which to request additional data. Phase I includes a web-based application, secure data transfer protocols between researchers, IMS and registries, and use of a single record linkage software (Match*Pro) optimized for linkages between cancer registries and research cohorts. Phase I functionality has been completed. The Phase I web-based application also serves as a standard application used by nearly all registries for their Phase II registry/IRB application process.
- Phase II supports the researcher's process of applying to registries and their IRBs for release of individual-level cancer data on matched cases identified in Phase I. It includes the following efficiencies:
- A Templated IRB/Registry Application (a.k.a. the phase I application) adopted for use by nearly all registries,
- Compilation of any required registry applications or agreements,
- A Templated Data Use Agreement used in lieu of many of the state-specific agreements,
- A dedicated Central IRB (CIRB) that reviews VPR linkage requests for local IRBs that have ceded their review to the CIRB. An integrated data entry and tracking system to monitor the status of a study request across multiple cancer registries.
Resources to Streamline the Data Release Application and Review Process
Phase II of the VPR-CLS will streamline the process of applying for release of individual-level data on matched cases by offering registries and state/local IRBs optional use of the following resources:
- Templated Forms: NAACCR has led efforts to create the following templated forms that can be used in lieu of the state-specific forms:
- Templated IRB/Registry Application (TIRA): The TIRA is a single application that includes common questions from across 50+ individual registry and IRB applications. The majority of VPR-participating registries have adopted use of the TIRA in lieu of their state-specific applications. The TIRA is filled out once during the initial Phase I VPR application process and is then shared with registries/IRBs for review during Phase II.
- VPR Templated Data Use Agreement (VPR DUA): The VPR DUA is a single DUA, with standard terms and conditions, developed by the NAACCR Templated DUA Task Force and vetted with registries, researchers, and legal teams. Over half of the VPR-participating registries have adopted the VPR DUA has been adopted in lieu of their state-specific DUAs.
- Central IRB(CIRB): Funded by NCI, the Biomedical Research Alliance of New York (BRANY) serves as the CIRB for multi-site minimal risk linkage studies coming through the VPR. The CIRB performs human subjects review in lieu of local/state IRBs that have entered into a reliance agreement with BRANY. Nearly 70% of the local/state IRBs have switched to using the CIRB and membership is growing.
Linkage Methodology
All VPR-CLS linkages are performed using the record linkage software, Match*Pro, developed by IMS. Match*Pro conducts probabilistic linkage based on the Fellegi and Sunter model. The following variables are used, as available, to link the study file with the registry file: First name, middle name, last name, maiden name, date of birth, social security number, telephone number, gender, and street address. After probabilistically identifying potential matches, deterministic filters classify each linked pair as a match, non-match, or uncertain.
Overview of VPR-CLS Workflow
Phase I: Application to use VPR-CLS to link with registries behind their firewall and receive aggregate match counts only (no state IRB or registry review needed).
Anticipated Timeline: Researchers can expect the application review process to take 2-4 weeks. After approval and upload of a validated, edited study file, the researcher can expect to receive registry match counts within 6-8 weeks depending on the list of studies in the queue for linkage.
- Researcher submits the online VPR-CLS application and supporting documents. Supporting documents include the current IRB determination, approved study protocol, consent form or waiver of consent, investigator’s curriculum vitae, and signed DUA with IMS.
- NAACCR reviews application for completeness and resolves any issues with researcher.
- Research Review Committee (RRC), made up of seven representatives from cancer registries and key stakeholder organizations, reviews application and researcher notified of decision.
- Researcher creates, edits, and uploads an encrypted study linkage file to the VPR-CLS in accordance with established file specifications and editing software.
- Encrypted study file is validated and posted for pre-test linkage with select registries.
- All registries perform linkage behind their firewalls using Match*Pro and standard linkage logic.
- Registries create and upload an aggregate match count report to the VPR-CLS that includes the number of high quality and uncertain matches by diagnosis year (no patient records).
- VPR-CLS reads the reports and presents researcher with the match counts.
Phase II: Application for release of individual-level data on matched cases identified during Phase I.
Anticipated Timeline: IRB/Registry approval and release of data will vary based on the review process in each state and whether the TIRA (Templated IRB/Registry Application) and CIRB can be utilized.
- During Phase I, Researcher already submitted the TIRA and supporting documents for NAACCR review and once all registries have uploaded their match counts, the request proceeds to the next step.
- Researcher reviews registry match counts and their adoption of VPR-CLS efficiencies (TIRA, VPR DUA, and CIRB) to inform selection of registries for Phase II, thereby finalizing the TIRA.
- BRANY CIRB reviews the TIRA and enters approval for the relying registry IRBs in the VPR-CLS.
- Researcher completes the remaining application materials and monitors the status of the request by leveraging the VPR-CLS list of additional required forms and agreements, interactive tracking system, and automated notifications and reminders.
- Registries and local/state IRBs, as appropriate, review the study and sign agreements.
- Upon approval and full execution of agreements, registries create a file of individual-level data for matched cases, including the Cohort ID and requested registry variables.
- Registries transmit the data file directly to the researcher through a secure site, independent of the VPR-CLS, as specified by either the researcher or the registry.
Data Security and Protections
The VPR-CLS provides a web-based portal through which researchers submit an application to use the system to link with registries. A Data Use Agreement is signed between the researcher and IMS prior to uploading any study data files containing patient identifiers to the secure data transmission service. The website uses Transport Layer Security, ensuring that communication and files transferred between a client and the IMS server are securely encrypted. All study data files are encrypted by the researcher (using Match*Pro) prior to uploading them to the VPR-CLS. All files uploaded to the VPR-CLS are first scanned for viruses and then stored on a secure server behind the IMS firewall. Only authorized full-time IMS staff can access the study files provided by researchers and all IMS staff have been trained in the handling of files that contain personal identifying information.
Once an uploaded encrypted study file has been reviewed and approved by IMS, it will be accessible for secure download by an authorized liaison from each of the participating registries. All registry liaisons are authenticated and verified by IMS prior to receiving access to the VPR-CLS. In addition, each registry has confirmed compliance with a list of common security protections described in the VPR-CLS Cancer Registry Security Protections document. Individually signed checklists are located on the About/Participating Registries tab and a summary of these protections across registries is available here.
Each registry will download the encrypted study file, perform the linkage behind their firewall, then upload a report of the aggregate match counts to the VPR-CLS. Once a registry has uploaded their aggregate match counts, the study file will no longer be accessible for download and the registry will be prompted to delete the study file and provide confirmation of destruction within the VPR-CLS. Once all registries have uploaded their match counts or the request goes into Phase II, the study file is removed from the VPR-CLS.
In Phase II, after all the participating registries have transmitted record-level data to the study requestor, the requestor is given the option to have each registry delete the linkage results file produced by Match*Pro. These registries are required to provide confirmation of data destruction within the VPR-CLS. IMS will also be prompted to delete the study file from the IMS server. All backups at IMS are removed after 1 year.
Registry Participation and Available Data for Linkage
Registry participation it the VPR-CLS is voluntary. As of 2022, 46 registries are participating representing 90% of the U.S. population (see map below). Details about the participating registries, including years of available data, population size, annual cancer counts, contact information, and security protections can be found here.
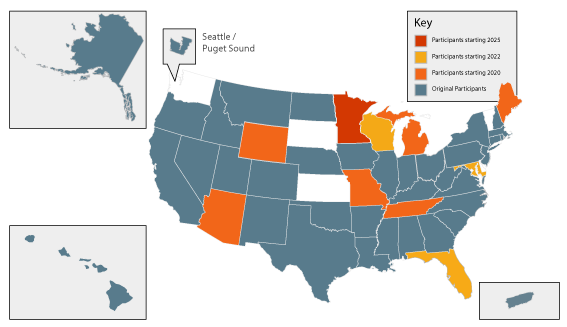
U.S. cancer registries have varying years of complete data available for linkage as noted on the NAACCR website. The majority of registries will include the most recent data in their VPR-CLS linkage file (e.g. In 2019, cancer cases diagnosed through the end of 2017 would be included). Cancer registries provide standardized, curated, population-based data on patient demographics, incidence, stage, treatment, and follow up.
Acknowledgements
The VPR-CLS development team would like to acknowledge the role of the National Heart, Lung, and Blood Institute and its BioLINCC Program in the approach used in the development of this website. The VPR-CLS code was based on the success of the BioLINCC framework, which shares many of the same advanced features and a well-defined yet flexible workflow for request submission and fulfillment.
BioShare is developed and maintained by Information Management Services, Inc.
